About two weeks ago, we paid a visit to our campus’ own BCA Green Mark (Platinum) certified (BCA, 2018) net-zero energy building at SDE4. While SDE4’s energy-saving, energy-producing, and innovative construction measures were intriguing, what got my water-obsessed brain fired up were its water recycling facilities.
Under BCA’s Green Mark Criteria (BCA, 2016), points are awarded where non-potable water scavenged is used to reduce potable water usage. SDE4 achieves this using a rainwater harvesting system, where two-thirds is used for flushing and irrigation of gardens, and the remaining is fed through a simulated wetland called a bio-retention basin before being discharged into sewers, as seen below.
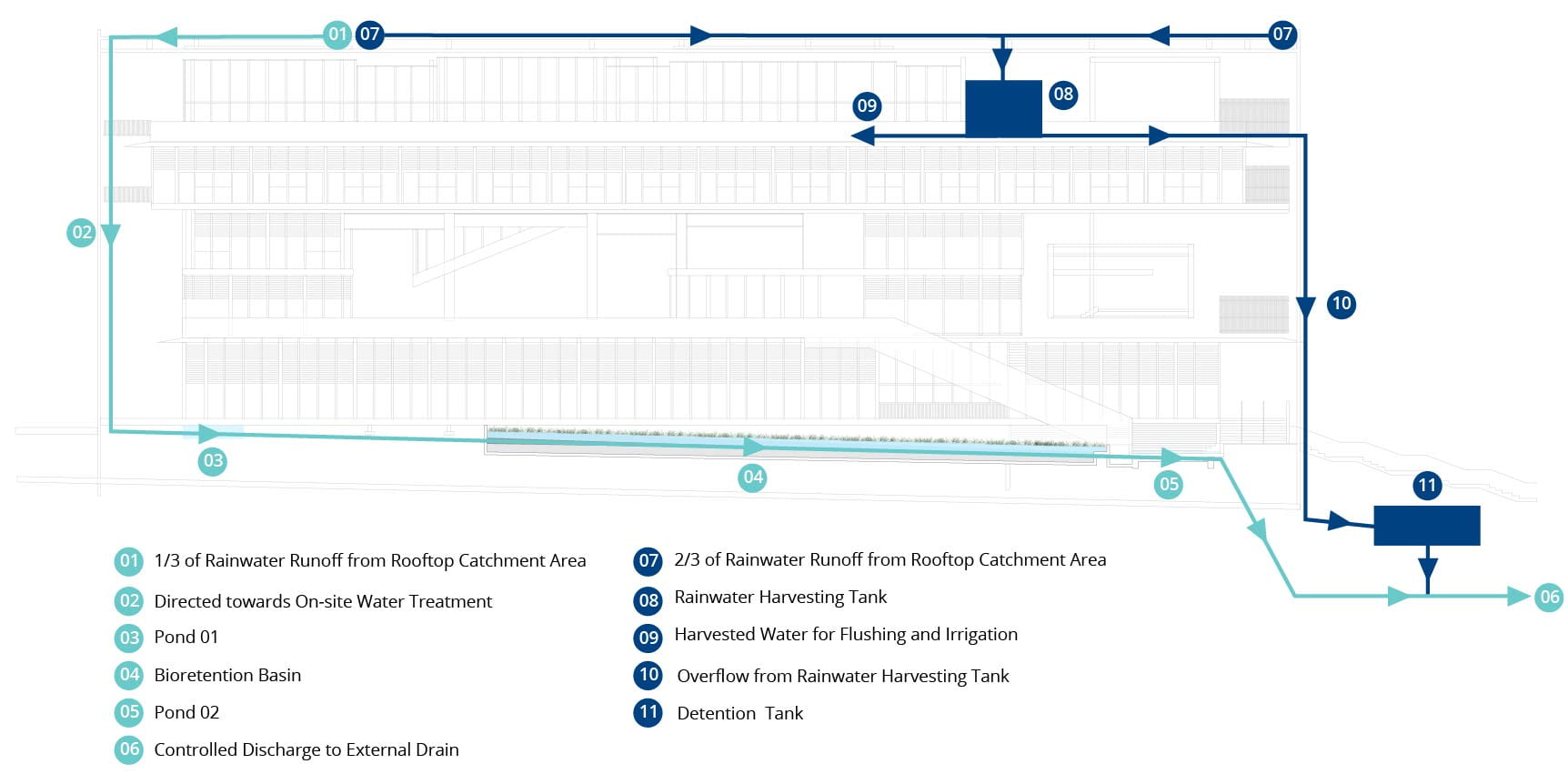
Schematic of SDE4’s rainwater harvesting and recycling system. Source: https://www.learningtrail.io/WATER_TRAIL/WATER_TRAIL

Diagram of SDE4’s Bio-retention Basin. Source: https://www.learningtrail.io/WATER_TRAIL/BIO_RETENTION_BASIN

The Bio-retention Basin has a similar design to a Vertical Flow Wetland that is used for sewage treatment. Its abilities include reducing high BOD, suspended solids and pathogenic loads, and converting ammonia to nitrogenous oxides by wetland microbes. (Tilley, Lüthi, Morel, Zurbrügg, & Schertenleib, 2008). Source: http://www.iwa-network.org/wp-content/uploads/2016/06/Compendium-Sanitation-Systems-and-Technologies.pdf
This essentially makes SDE4 one of our 4 national taps: a mini catchment area!
While walking around the learning trails, something under the sink outside the fifth-floor design studios caught my eye.

A small biological treatment tank I found outside the Design Studio.

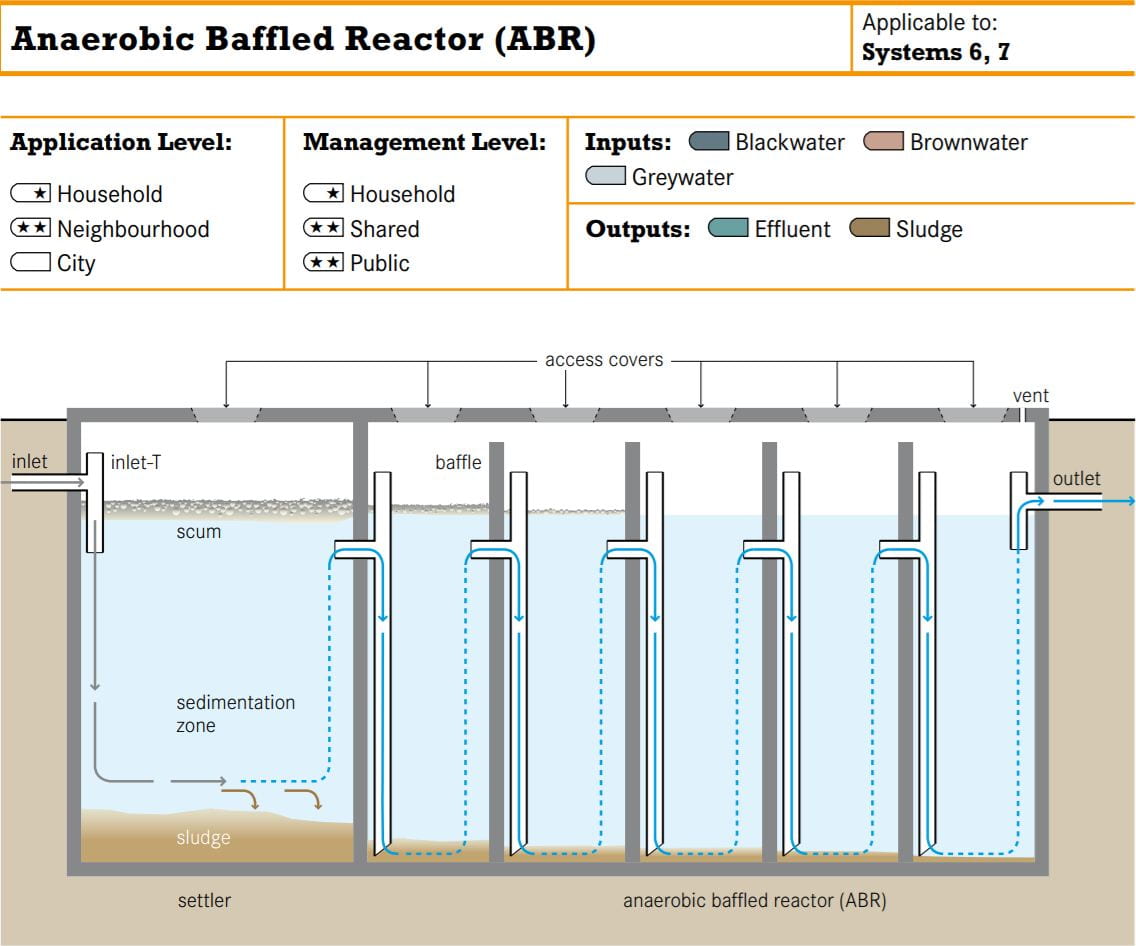
Alternative baffles can be seen, giving wastewater pollutants a higher retention time inside the reactor for the bacteria do decompose the pollutants.
Since that was the first bioreactor I have seen in person, I couldn’t help but open it.

There are visible improvements in water clarity and amount of scum produced as the water proceeds through the bio-reactor.
As vile as it looks, the smell was not particularly objectionable and similar to a mangrove swamp, hinting its modus operandi.
From what I can surmise, the reactor design incorporates elements from an anaerobic baffled bioreactor as pictured below.
Exposure to aerobic conditions above the surface and anoxic conditions below the surface might increase the diversity of the microbial community in the reactor, thereby expanding the range of pollutants it can handle. This might be appropriate since Design Studio personnel handle resins and aerosol solvents that are prohibited under the SDA effluent discharge limits. Unfortunately, I couldn’t get clarification from the SDE on this instalment.
With the building’s energy budget as constrained as it is, this bioreactor fits right in since the bacteria do all the work without any extra energy (Tilley et al., 2008)! However, without any sort of disinfection system or a clarification tank to remove any remaining pathogens and microbes, recycling the resulting effluent might not be so wise (Tilley et al., 2008).
Hence, I decided to look for low-energy alternatives for water recycling solutions; and what I found was a two-for-one deal!
Several studies have investigated the applicability of incinerator fly ash and bottom ash as an adsorbent for wastewater pollutants. Conventionally, this is done using activated carbon, which is costly (Ahmaruzzaman & Gupta, 2011). Conversely, fly and bottom ash are produced by the truckload every day. They have proven themselves against a wide variety of simulated wastewaters including pharmaceuticals & petrochemicals (Sunil J. Kulkarni, Sonali R. Dhokpande, 2013) and dyes (Gupta et al., 2005) which may be produced by the Design Studio personnel. The resulting effluent could contribute to the non-potable water demands of the building.
These techniques have not been rigorously tested and applied industrially, unlike energy-intensive reverse osmosis or activated carbon (Ahmaruzzaman & Gupta, 2011). However, SDE4 provides an amazing platform for pilot scale fixtures to be tested such that they may be feasibly applied to other buildings to spur low-energy water recycling and self-sufficiency!
References:
Ahmaruzzaman, M., & Gupta, V. K. (2011). Rice husk and its ash as low-cost adsorbents in water and wastewater treatment. Industrial and Engineering Chemistry Research. https://doi.org/10.1021/ie201477c
BCA. (2016). GREEN MARK FOR NON-RESIDENTIAL BUILDINGS NRB: 2015 including Hawker Centres, Healthcare Facilities, Laboratory Buildings and Schools. Building and Construction Authority Green Mark, 73. Retrieved from https://www.bca.gov.sg/GreenMark/others/Green_Mark_NRB_2015_Criteria.pdf
BCA. (2018). BCA Awards 2018. Building and Construction Authority Green Mark, 85. Retrieved from https://www.bca.gov.sg/GreenMark/others/gm2018.pdf
Gupta, V. K., Ali, I., Saini, V. K., Van Gerven, T., Van Bruggen, B. Der, & Vandecasteele, C. (2005). Removal of dyes from wastewater using bottom ash. Industrial and Engineering Chemistry Research. https://doi.org/10.1021/ie0500220
Sunil J. Kulkarni, Sonali R. Dhokpande, J. P. K. (2013). Studies On Flyash As An Adsorbent For Removal Of Various Pollutants From Wastewater. International Journal of Engineering Research & Technology, 2(5), 1190–1195. Retrieved from https://www.ijert.org/research/studies-on-flyash-as-an-adsorbent-for-removal-of-various-pollutants-from-wastewater-IJERTV2IS50668.pdf
Tilley, E., Lüthi, C., Morel, A., Zurbrügg, C., & Schertenleib, R. (2008). Compendium of Sanitation Systems and Technologies. In Development. Retrieved from http://www.iwa-network.org/wp-content/uploads/2016/06/Compendium-Sanitation-Systems-and-Technologies.pdf