Today’s World Antibiotic Awareness Week blog post comes from Richard Smith, Professor of Health System Economics at the London School of Hygiene & Tropical Medicine. His research focuses on how a country’s health system interacts with other parts of the economy. In this post, he talks about the wide-ranging costs of antimicrobial resistance to individuals and society, and how economists think about the problem of antimicrobial resistance.
Antibiotic resistance (AMR) has reasserted itself on the national and international agenda as a critical threat to public health and health systems. Although AMR has a biological mechanism, it is fundamentally a social problem of profligate use (and abuse), and building modern health systems that are heavily dependent on antibiotics. AMR undermines the very foundations of modern health care, from joint replacements to chemotherapy, threatens to reverse the decline in mortality and morbidity from infectious diseases, jeopardises animal health and welfare and poses potentially crippling financial impacts.
These financial impacts are wide ranging. AMR results in an extensive increase in costs to the patient and family, the hospital, and society, due to the need for the use of more expensive drugs for second line treatment, more tests and much longer stays in hospital, as well as longer sick leave, or even premature death. Treatment failure is the main contributor to increased costs and can lead to: additional investigations such as laboratory tests and X-ray examinations; additional or alternative treatments, often much more expensive than drugs used to treat infections caused by sensitive organisms; additional side-effects from more toxic treatments, which have to be managed; longer hospital stay; longer time off work; reduced quality of life; greater likelihood of death due to inadequate or delayed treatment; increased burden on family of infected individual; increases in private insurance coverage additional cost for hospital when hospital- acquired infection occurs and infection control procedures are required; increased overall healthcare expenditure, including costs of combating transmission; increased cost of disease surveillance; increased costs to firms of absenteeism; possible increase in product prices due to increased costs to firms. Depending what is counted, these costs vary enormously from $5 to $55,000 per patient episode. But whatever they are, they are certainly an under-estimate as they tend to look at the very immediate and direct costs, such as those indicated above.
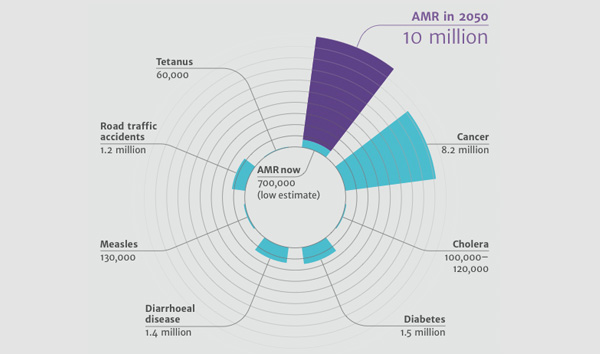
However, an even greater economic burden lies in the future and will affect generations to come, when the consequences of current use (not just ‘misuse’) of antibiotics could lead to the loss of all the advantages in medical care that they have brought about. Advanced surgical procedures and cancer chemotherapy might be impossible to perform, resulting in enormous costs, economic as well as human. As an example, we estimated the consequences of having no antibiotics for patients having a total hip replacement, and find that compared to current infection rates of about 0.5-2%, without antibiotics the rate of postoperative infection could be 40-50%, with a mortality rate of about 30%. Antibiotics are the cornerstone of modern medicine that revolutionized medical care during the last half of the previous century. From cradle to grave the role of antibiotics in safeguarding the overall health of human societies is pivotal; they are given, for example, as standard to prevent iatrogenic infection in surgical care, to women delivering by caesarean section, and to those having cancer treatment. The costs of antibiotic resistance relate to the loss of these benefits and associated treatment possibilities at every stage of human life. Thus, in order to calculate the full economic burden of antibiotic resistance we have to consider the burden of not having antibiotics at all, which at the extreme would probably collapse the entire modern medical system.
Image credit: Jim O’Neill’s Review on Antimicrobial Resistance for the UK Government.
But apart from trying to quantify the financial impacts of AMR, and assess the cost-effectiveness of policies and interventions to try and tackle AMR, economists have also worked on defining AMR itself. In economic terminology, resistance is a negative externality. That is, it has an undesirable effect on people other than the immediate consumer – in this case, the patient taking the antibiotic. This negative externality cross-sectional as it is imposed on people other than the patient, but also temporal in the sense that when the consequences of resistance have appeared a cost is also borne by the patient themselves at some point in the future. Why is this relevant? For two reasons. First, we are not looking at just impacting on ‘inappropriate use’ – resistance accrues with appropriate use also (in clinical terms, taking a drug that will impact on the disease). What we are looking for is what economists call ‘optimal’ use, where the costs (current and future, and including this externality) are balanced against the benefits. In some areas it means that it is quite possible that society should be denying clinically effective treatment, for example to self-limiting conditions such as sore throats, to ensure that the drugs are available for major illness, such a sepsis. Second, we are looking at containment of resistance, not eradication. As AMR is associated with antibiotic usage (appropriate or otherwise), and the interaction of microorganisms, people and the environment, it is therefore not eradicable, but will have to be managed.
This management means we will need to plan for a future with AMR, not one that tried to avoid or eradicate it. Yet considerable inertia remains regarding radical change in our stewardship of antibiotics; the same is true with climate change. And as with climate change, we can all do our bit. So, especially during World Antibiotic Awareness Week, a good start would be to follow three simple rules: (1) try not to take an antibiotic – most viruses and infections clear within a few days; (2) do not pressure your physician to prescribe an antibiotic; (3) take any antibiotic exactly as prescribed.