Hot Off the Press: Selective induction of alternatively spliced FynT isoform by TNF facilitates persistent inflammatory responses in astrocytes

In this study led by Michelle Tan, and following hot on the heels of our previous paper showing association of the FynT isoform with astrocyte activation in AD (Lee et al, 2016), we demonstrated selective FynT induction in murine cortex and primary astrocyte culture after prolonged exposure to inflammatory stimulants, suggesting that FynT may mediate persistent neuroinflammation. To delineate the functional role of astrocytic FynT in association with TNF-mediated inflammatory responses, immortalized normal human astrocytes (iNHA) stably expressing FynT kinase constitutively active (FynT-CA) or kinase dead (FynT-KD) mutants were treated with TNF and compared for inflammatory responses using high-throughput real-time RT-PCR and Luminex multi-analyte immunoassays. FynT-CA but not FynT-KD mutant exhibited drastic induction of proinflammatory cytokines and chemokines after prolonged exposure to TNF, which could be attenuated by treating with Fyn kinase inhibitor PP2 or silencing via FynT-specific DsiRNA. FynT kinase activity-dependent induction of PKCδ expression, PKCδ phosphorylation, as well as NFκB activation was detected at the late phase but not the early phase of TNF signaling. In conclusion, selective FynT induction by TNF may facilitate persistent inflammatory responses in astrocytes, which is highly relevant to chronic neuroinflammation in neurodegenerative diseases including but not limited to AD.
References
Lee C, Low CY, Wong SY, et al. (This paper).
Lee C, Low CY, Francis PT, Attems J, Wong PT, Lai MK, Tan MG (2016) An isoform-specific role of FynT tyrosine kinase in Alzheimer’s disease. J Neurochem 136:637-50.

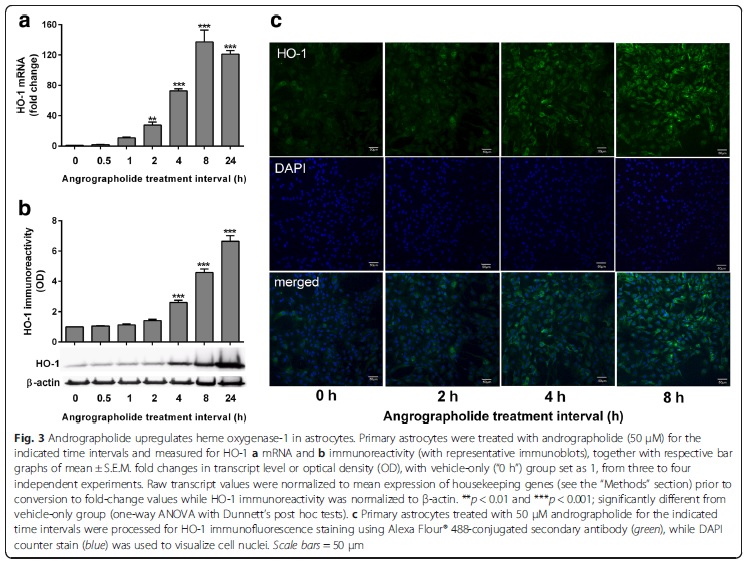 Andrographolide is the major labdane diterpenoid originally isolated from Andrographis paniculata and has been shown to have anti-inflammatory and antioxidative effects. However, there is a dearth of studies on the potential therapeutic utility of andrographolide in neuroinflammatory conditions. Continuing our previous work showing the anti-chemokine effects of andrographolide (Wong et al. 2014; 2016), we investigated the mechanisms underlying andrographolide’s effect on the expression of anti-inflammatory and antioxidant heme oxygenase-1 (HO-1) in primary astrocytes. Measurements of the effects of andrograholide on antioxidant HO-1 and its transcription factor, Nrf2, include gene expression, protein turnover, and activation of putative signaling regulators, p38 and ERK, were carried out. We found that andrographolide potently activated Nrf2 and also upregulated HO-1 expression in primary astrocytes. Interestingly, andrographolide’s effects on Nrf2 seemed to be biphasic, with acute (within 1 h) reductions in Nrf2 ubiquitination efficiency and turnover rate, followed by upregulation of Nrf2 mRNA between 8 and 24 h. The acute regulation of Nrf2 by andrographolide seemed to be independent of Keap1 and partly mediated by p38 MAPK and ERK signaling. These data provide further insights into the mechanisms underlying andrographolide’s effects on astrocyte-mediated antioxidant, and anti-inflammatory responses and support the further assessment of andrographolide as a potential therapeutic for neurological conditions in which oxidative stress and neuroinflammation are implicated.
Andrographolide is the major labdane diterpenoid originally isolated from Andrographis paniculata and has been shown to have anti-inflammatory and antioxidative effects. However, there is a dearth of studies on the potential therapeutic utility of andrographolide in neuroinflammatory conditions. Continuing our previous work showing the anti-chemokine effects of andrographolide (Wong et al. 2014; 2016), we investigated the mechanisms underlying andrographolide’s effect on the expression of anti-inflammatory and antioxidant heme oxygenase-1 (HO-1) in primary astrocytes. Measurements of the effects of andrograholide on antioxidant HO-1 and its transcription factor, Nrf2, include gene expression, protein turnover, and activation of putative signaling regulators, p38 and ERK, were carried out. We found that andrographolide potently activated Nrf2 and also upregulated HO-1 expression in primary astrocytes. Interestingly, andrographolide’s effects on Nrf2 seemed to be biphasic, with acute (within 1 h) reductions in Nrf2 ubiquitination efficiency and turnover rate, followed by upregulation of Nrf2 mRNA between 8 and 24 h. The acute regulation of Nrf2 by andrographolide seemed to be independent of Keap1 and partly mediated by p38 MAPK and ERK signaling. These data provide further insights into the mechanisms underlying andrographolide’s effects on astrocyte-mediated antioxidant, and anti-inflammatory responses and support the further assessment of andrographolide as a potential therapeutic for neurological conditions in which oxidative stress and neuroinflammation are implicated.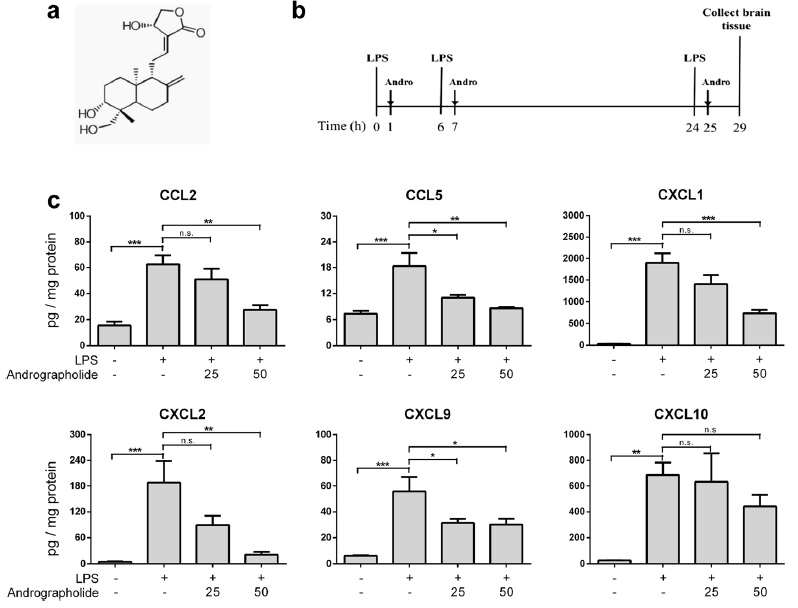
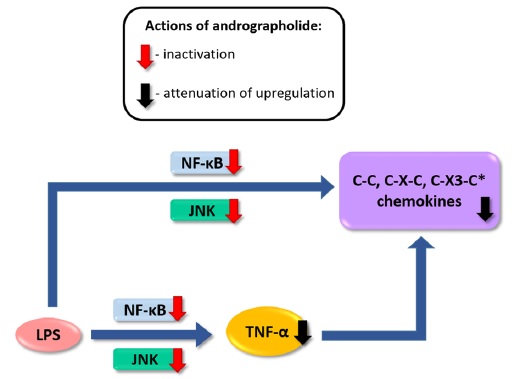 induced chemokine up-regulation both in mouse cortex and in cultured primary astrocytes were measured, including cytokine profiling, gene expression, and, in cultured astrocytes, activation of putative signaling regulators. We found that orally administered andrographolide significantly attenuated mouse cortical chemokine levels from the C-C and C-X-C subfamilies. Similarly, andrographolide abrogated a range of LPS-induced chemokines as well as tumor necrosis factor (TNF)-α in astrocytes. In astrocytes, the inhibitory actions of andrographolide on chemokine and TNF-α up-regulation appeared to be mediated by nuclear factor-κB (NF-κB) or c-Jun N-terminal kinase (JNK) activation. These results suggest that andrographolide may be useful as a therapeutic for neuroinflammatory diseases, especially those characterized by chemokine dysregulation.
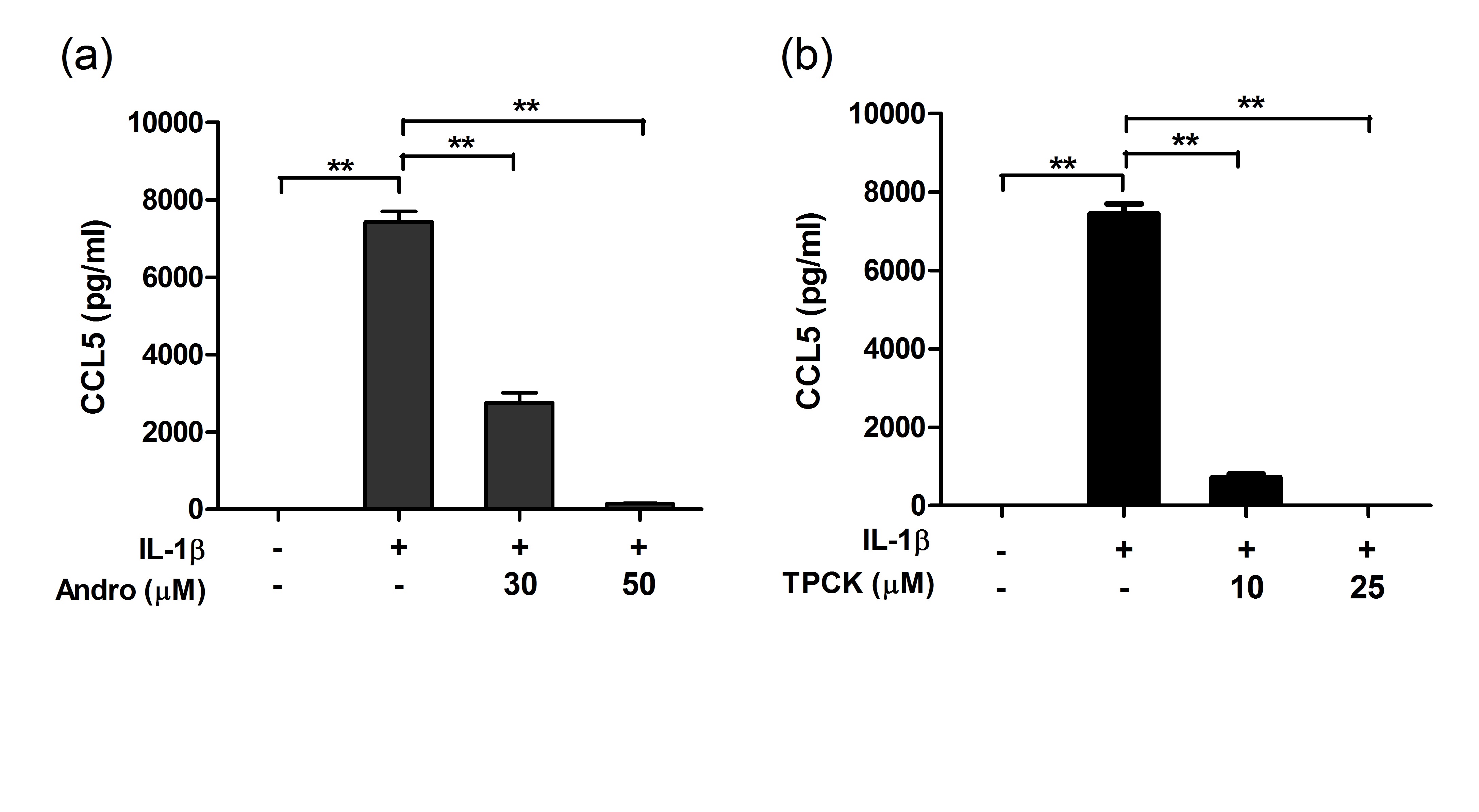
induced chemokine up-regulation both in mouse cortex and in cultured primary astrocytes were measured, including cytokine profiling, gene expression, and, in cultured astrocytes, activation of putative signaling regulators. We found that orally administered andrographolide significantly attenuated mouse cortical chemokine levels from the C-C and C-X-C subfamilies. Similarly, andrographolide abrogated a range of LPS-induced chemokines as well as tumor necrosis factor (TNF)-α in astrocytes. In astrocytes, the inhibitory actions of andrographolide on chemokine and TNF-α up-regulation appeared to be mediated by nuclear factor-κB (NF-κB) or c-Jun N-terminal kinase (JNK) activation. These results suggest that andrographolide may be useful as a therapeutic for neuroinflammatory diseases, especially those characterized by chemokine dysregulation.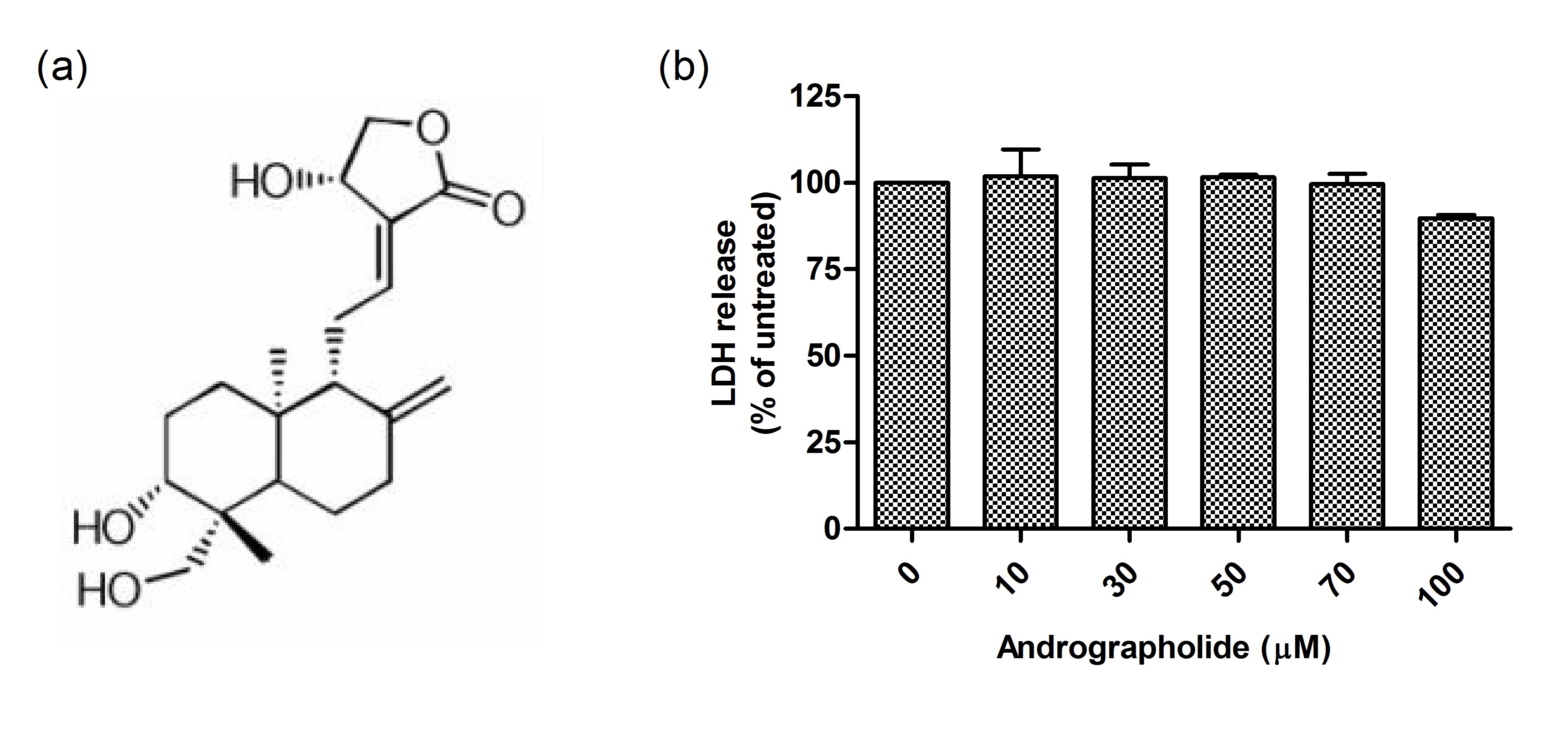 Andrographolide is a bioactive molecule isolated from Andrographis paniculata with anticancer and anti-inflammatory activities. In this study, we tested the effects of andrographolide on astrocyte-mediated neuroinflammatory responses. Cultured rat primary astrocytes were treated with proinflammatory cytokine interleukin 1β with or without pretreatment with andrographolide, and then processed for measurements of chemokine C-C motif ligand 5 (CCL5) and glial fibrillary acidic protein. The activation status of nuclear factor-κB activation that may underlie CCL5 upregulation was also measured. Andrographolide pretreatment was found to attenuate the upregulation of CCL5 and glial fibrillary basic protein as well as reduce the phosphorylation of nuclear factor-κB p65 and IκBα after interleukin 1β stimulation. These data suggest that andrographolide should be evaluated further as a therapeutic for central nervous system diseases characterized by astrocyte-mediated neuroinflammatory processes.
Andrographolide is a bioactive molecule isolated from Andrographis paniculata with anticancer and anti-inflammatory activities. In this study, we tested the effects of andrographolide on astrocyte-mediated neuroinflammatory responses. Cultured rat primary astrocytes were treated with proinflammatory cytokine interleukin 1β with or without pretreatment with andrographolide, and then processed for measurements of chemokine C-C motif ligand 5 (CCL5) and glial fibrillary acidic protein. The activation status of nuclear factor-κB activation that may underlie CCL5 upregulation was also measured. Andrographolide pretreatment was found to attenuate the upregulation of CCL5 and glial fibrillary basic protein as well as reduce the phosphorylation of nuclear factor-κB p65 and IκBα after interleukin 1β stimulation. These data suggest that andrographolide should be evaluated further as a therapeutic for central nervous system diseases characterized by astrocyte-mediated neuroinflammatory processes.