Acid rain is yet another one of the long lists of air pollution consequences, and is broadly classified under the scientific term acid deposition. It is a serious environmental threat that if left unchecked, would result in severe environmental damage to ecological systems and affect human lives. Some regions that have been historically plagued by acid rain include the Black Triangle – Czech Republic, Germany and Poland as well as the eastern United States. Nowadays, it has become a common phenomenon worldwide, especially in rapidly industrializing countries like China and India.
Acid rains are typically associated with poor air quality and pollution. So what is acid rain and how is it formed? Why are its impacts so concerning?
What is acid rain?
Acid rain describes any form of precipitation that contains significant amounts of nitric and sulfuric acids, causing the pH levels to be between 4.2 and 4.4. Acid rain can come in two forms – wet deposition and dry deposition. Wet deposition refers to rain, hail, snow and sleet, whereas dry deposition takes place in the absence of moisture such as via gasses and particles. Both forms can be transported by wind currents over long distances.
How does it come about?
Although acid rain can be caused by natural processes like volcanic eruptions, it is more often primarily due to anthropogenic activities. The burning of fossil fuels for energy generation as well as vehicular emissions generates sulfur dioxide and nitrogen oxides, which then combine with moisture in the atmosphere to form acidic chemicals. These chemicals hence fall back to Earth via acid rain. The diagram in Fig 1 explains the entire process.
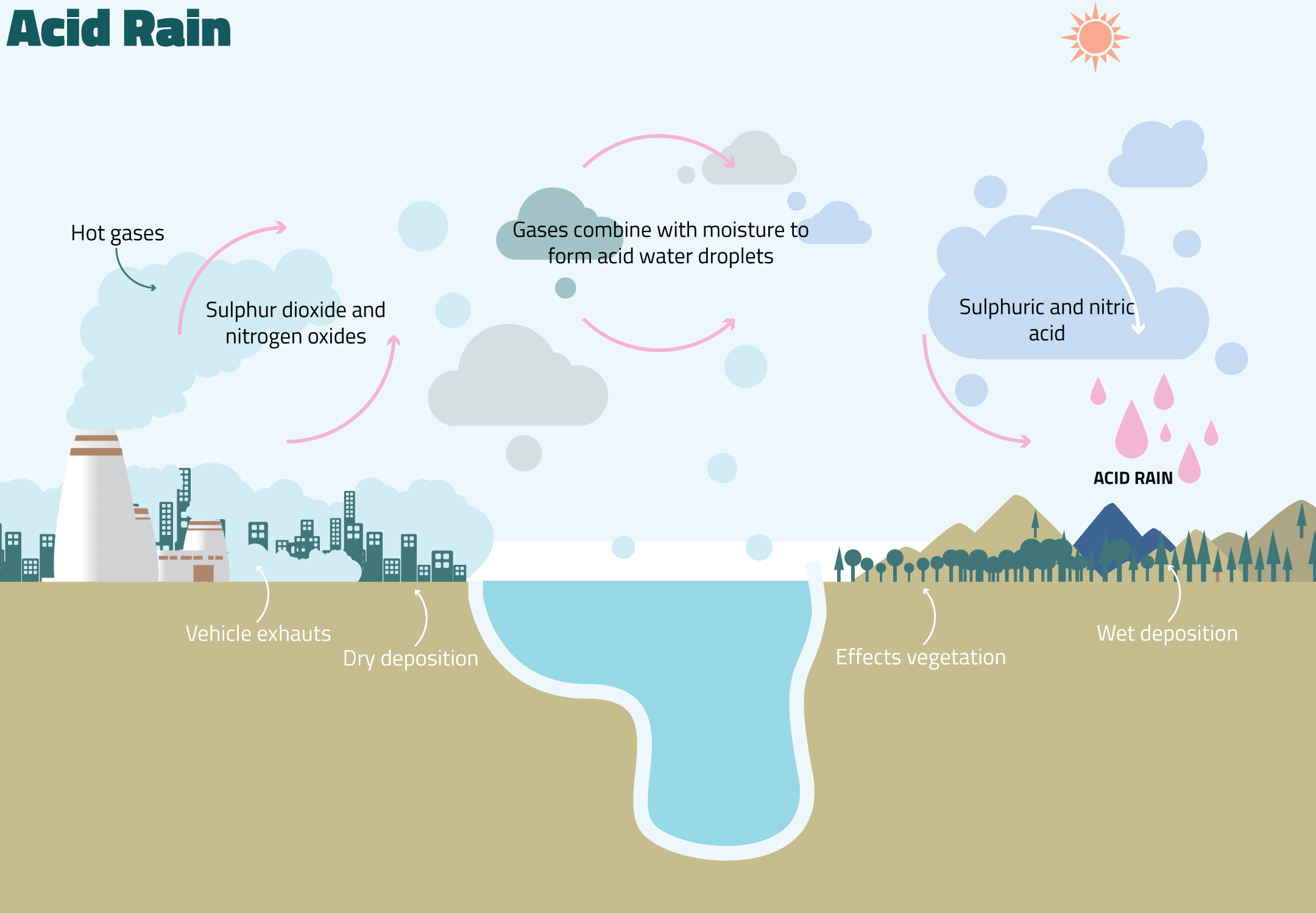
Fig 1: Formation of acid rain (SEPA, 2015)
What are the impacts of acid rain?
Acid rain triggers a series of inorganic and biochemical reactions in many ecosystems. Firstly, it results in the corrosion of buildings and structures, especially those with metal surfaces, leading to higher maintenance costs. Corrosive effects can also be observed on rocks made of limestone and marble, which disintegrate and crumble over time. Historic sculptures that hold ancient heritage and cultural meanings are therefore destroyed by acid rain.

Fig 2: A gargoyle corroded by acid rain (Source: Barbieri, 2006)
Secondly, acid rain also brings harmful impacts to nature and wildlife. It acidifies water bodies and alters the natural pH levels of these aquatic habitats. Wildlife species that are unable to adjust to these new conditions would thus perish as their sources of food and homes disappear. For instance, lower pH levels increase concentrations of dissolved metals in water which is toxic to fish, reducing their population. Ecosystem functions are also impaired, which sets off a chain reaction that ultimately affects mankind. Furthermore, acid rain also leaches calcium from forest soils and changes forest plant biodiversity. It allows aluminum to accumulate in the soil which will weaken roots and reduces their capacity to draw water. In mountainous regions that experience acid fog, the trees are stripped of nutrients (Fig 3) and are often bare and dead.

Fig 3: What acid rain does to trees (Source: YPTE, n.d.)
Thirdly, acid rain also has adverse impacts on humans. Inhaling toxic particles during an acid rain event can cause eye and skin irritation, dental erosion, and respiratory illnesses such as chronic bronchitis.
Given all the negative socio and environmental consequences associated with acid rain, it is pertinent for mankind to scale down on actions that result in this phenomenon. If you are interested to know more about acid rain, check out this short video by National Geographic: https://www.nationalgeographic.com/environment/article/acid-rain
Bibliography
Bartels, S. F., Gendreau-Berthiaume, B., & Macdonald, S. E. (2019). The impact of atmospheric acid deposition on tree growth and forest understory vegetation in the Athabasca Oil Sands Region. Science of the Total Environment, 696: 133877.
Echolls, T. (2018, April 30). What Place in the World Receives the Most Acid Rain? Retrieved from Sciencing: https://sciencing.com/place-world-receives-acid-rain-23289.html
EPA. (2022, June 24). What is Acid Rain? Retrieved from United States Environmental Protection Agency: https://www.epa.gov/acidrain/what-acid-rain
ESA. (2000). Acid Deposition. Retrieved from Ecological Society of America: https://www.esa.org/wp-content/uploads/2012/12/aciddeposition.pdf
Fischer, K. (2022, November 21). What is Acid Rain? Retrieved from webMD: https://www.webmd.com/lung/acid-rain-what-is-it
Goodwall. (202, June 13). How Does Acid Rain Affect The Environment? Here Are the Harmful Effects. Retrieved from Goodwall Blog: https://www.goodwall.io/blog/how-does-acid-rain-affect-the-environment/
Kielmas, M. (n.d.). Natural Causes of Acid Rain. Retrieved from Seattle PI: https://education.seattlepi.com/natural-causes-acid-rain-4613.html
Knotková, D., & Bartoň, K. (1992). Effects of acid deposition on corrosion of metals. Atmospheric Environment. Part A. General Topics, 26(17): 3169-3177.
SEPA. (2015). Pollution and the Environment. Retrieved from Learning About Air Quality: http://www.learnaboutair.com/science/section_05.html

Leave a Reply