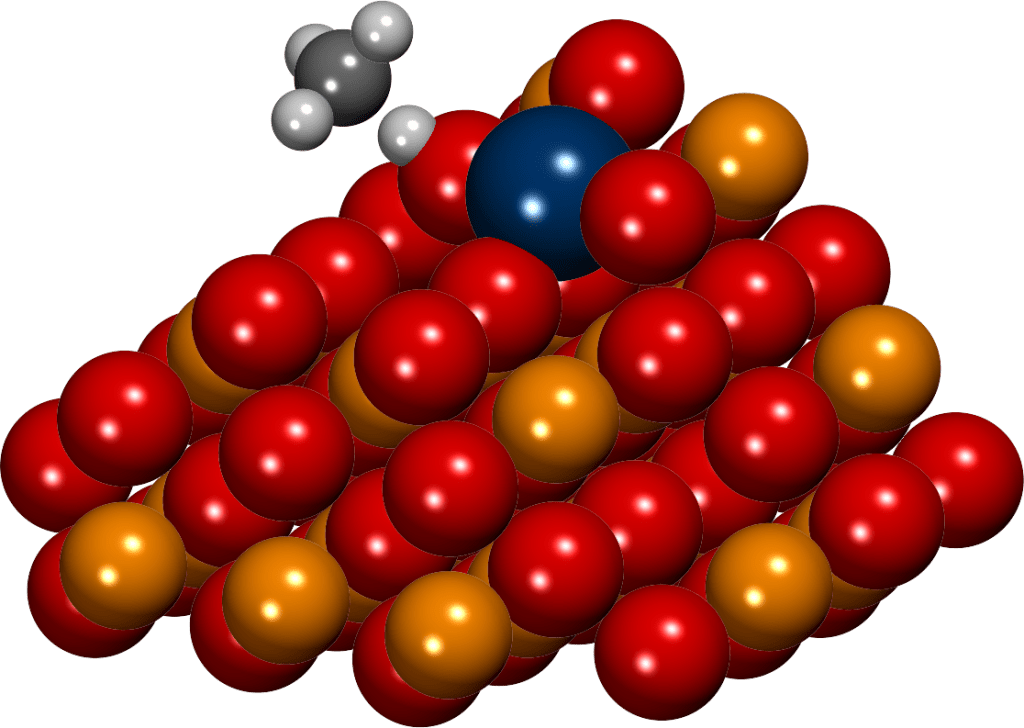
Nanoparticle-support interactions

Although nanoparticle-support interactions have been long known to have a crucial effect on catalytic activity, their experimental characterization remains extremely challenging. In our group, we simulate highly sophisticated and realistic models of transition metal nanoparticles on oxide supports using density functional methods. Such simulations reveal how oxide supports affect the properties and chemical activity of metal particles, for example, via nanoparticle-support charge transfer. Moreover, we are able to directly investigate the elusive properties of the active sites on the perimeter of nanoparticle-support interfaces, which often have extraordinary catalytic activity.
Diversity of platinum-sites at platinum/fullerene interface accelerates alkaline hydrogen evolution, Chen, Aliasgar, Zamudio, Zhang, Zhao, Lian, Wen, Yang, Sun, Kozlov, Chen, Wang, Nat. Commun. 2023, 14, 1711
The role of metal/oxide interfaces for long range metal particle activation during CO oxidation, Suchorski, Kozlov, Bespalov, Datler, Vogel, Budinska, Neyman, Rupprechter, Nat. Mater. 2018, 17, 519
Counting Electrons on Supported Nanoparticles, Lykhach, Kozlov, Skála, Tovt, Stetsovych, Tsud, Dvořák, Johánek, Neitzel, Mysliveček, Fabris, Matolín, Neyman, Libuda, Nat. Mater. 2016, 15, 284
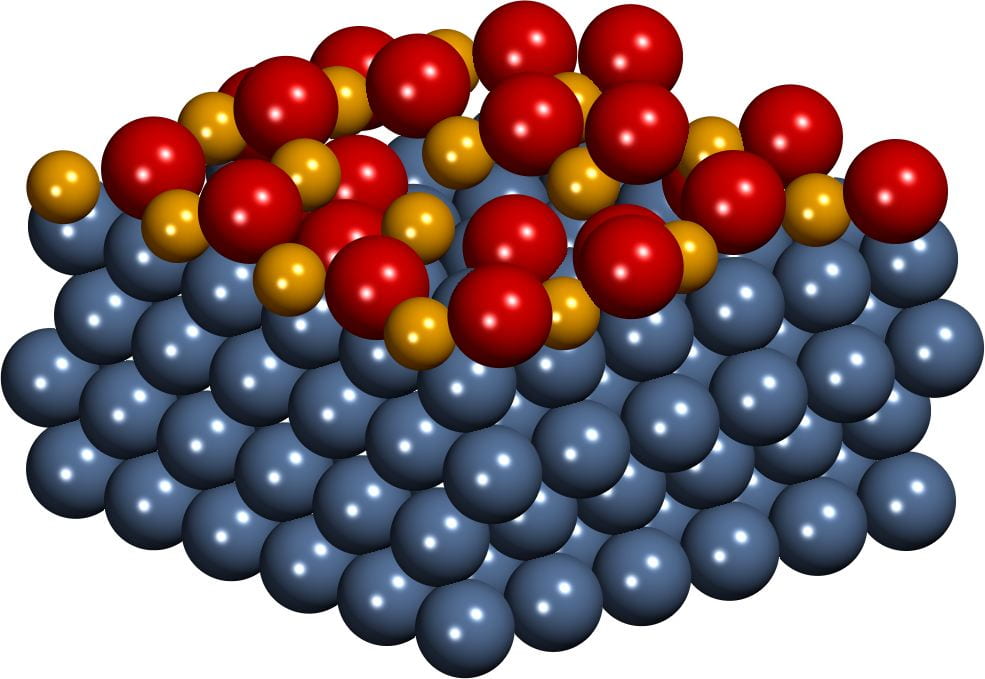
Oxide films on metal surfaces
Recent developments in catalyst preparation techniques enabled the synthesis of catalysts in the form of oxide films on metal surfaces that are sufficiently stable for industrial applications. Intricate interactions between oxide films and metal supports may result in unusual oxidation states of the atoms composing the film and unique catalytic properties. Using density functional simulations, we investigate how the charge transfer between the oxide films and the metallic supports affects the composition, the reducibility, and ultimately the catalytic activity of the films under reaction conditions.
Role of Oxidized Mo Species on the Active Surface of Ni−Mo Electrocatalysts for Hydrogen Evolution under Alkaline Conditions, Bau, Kozlov, Azofra, Ould-Chikh, Emwas, Idriss, Cavallo, Takanabe, ACS Catal, 2020, 10, 12858
Turning a methanation Co catalyst into an In-Co methanol producer, Bavykina, Yarulina, Al Abdulghani, Gevers, Hedhili, Miao, Ramirez, Pustovarenko, Dikhtiarenko, Cadiau, Aguilar Tapia, Hazemann, Kozlov, Ould-Chikh, Cavallo, Gascon, ACS Catal. 2019, 9, 6910
Alloy nanoparticles

Alloying has been demonstrated as a remarkably successful strategy for the discovery and rational design of new catalysts. Alloying can help by substituting precious noble metals with earth-abundant elements and generating new materials with unique properties. The properties of nanoalloys can be tuned to a large extent for a specific application because they strongly depend on the concentration and ordering of alloy components. In our group, we develop advanced computational approaches to investigate the chemical ordering of components within the alloy nanocrystallites. The developed methods are applied to study alloy catalysts for the most societally important reactions, such as methane conversion and electrochemical water splitting.
In-operando elucidation of bimetallic CoNi nanoparticles during high-temperature CH4/CO2 reaction, AlSabban, Falivene, Kozlov, Aguilar-Tapia, Ould-Chikh, Hazemann, Cavallo, Basset, Takanabe, Appl. Cat. B 2017, 213, 177
Surface composition changes of CuNi-ZrO2 during methane decomposition: An operando NAP-XPS and density functional study, Wolfbeisser, Kovács, Kozlov, Föttinger, Bernardi, Klötzer, Neyman, Rupprechter, Catal. Today, 2017, 283, 134
How to determine accurate chemical ordering in several nanometer-large bimetallic crystallites from electronic structure calculations, Kozlov, Kovács, Ferrando, Neyman, Chem. Sci. 2015, 6, 3868
Single-atom catalysts
Single-atom catalysts revolutionized heterogeneous catalysis by achieving 100% atomic efficiency and forming precisely defined active sites. Moreover, such catalysts may break scaling relations in heterogeneous catalysis and exhibit unique catalytic properties. At the same time, stabilizing single-atom catalysts under reaction conditions remains a formidable challenge due to their propensity to convert into subnanometer clusters. Our studies focus on the changes in the charge state and active site structure in single-atom catalysts, which sheds light on their deactivation mechanisms during catalytic reactions. Our simulations also allow us to compare the activity of single-atom and conventional nanoparticle catalysts in a given reaction.
Engineering nanoscale H supply chain to accelerate methanol synthesis on ZnZrOx, Lee, Mendes, Jeon, Song, Dickieson, Anjum, Chen, Yang, Yang, Choi, Kozlov, Yan, Nat. Commun. 2023, 14, 819
Atomic Pd-promoted ZnZrOx solid solution catalyst for CO2 hydrogenation to methanol, Lee, Anjum, Araújo, Mondelli, He, Furukawa, Pérez-Ramírez, Kozlov, Yan, Appl. Cat. B 2022, 304, 120994