Post-antibiotic world: the antibiotic apocalypse
Quek Sze Yang, Ngee Ann Polytechnic
Introduction
A grim and dreadful future is looming – a future where one of the most widely used medicines to treat diseases is rendered useless. Soon, common surgical procedures such as the removal of the appendix could be lethal. Even a simple cut to your finger could become life threatening. Sounds scary enough? This is not a plot adapted from a science fiction book. It is a future without antibiotics in the post-antibiotic world.
The discovery of antibiotics has significantly contributed to the control of infectious diseases, reducing the associated morbidity and mortality rate in both humans and animals. However, the prevalence of antimicrobial resistant bacteria (ARB) can limit the efficacy of antibiotics.
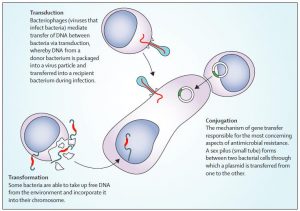
ARB emerge as a result of the bacteria’s ability to counter the effects of antimicrobial drugs such as antibiotics. There are various methods of transmission of resistance. For microorganisms, genetic material can be transferred via three main routes, as summarized in Figure 1. Despite being a natural phenomenon, ARB has propagated due to various reasons such as inadequate regulation along with the misuse of antimicrobial medication (WHO, 2016). It has a serious burden on society, ranging from substantial economic costs to increased morbidity and mortality rates (OECD, 2015).
Sir Alexander Fleming, the man behind the discovery of penicillin, in 1946, had already warned of the threat of antibiotic resistance back (Bartlett, Gilbert and Spellberg, 2013). Despite his warnings pertaining to overuse, antibiotics are still being overprescribed worldwide (The antibiotic alarm, 2013)
Consequences of Antibiotic Resistance to the human society
The antibiotic resistance crisis has been noted to be dire and have detrimental effects to society (WHO, 2014). Moving forward, non-lethal diseases will start to take longer to resolve. Additionally, lethal diseases will become more debilitating and fatal. These adverse consequences will have a significant effect on human society.
As disease severity, strain virulence and host vulnerability increase, the magnitude of these detrimental outcomes will become more pronounced. In the case of gonorrhoea for example, the high prevalence of N. gonorrhoeae strains that are impervious to most antibiotics had led to problems such as treatment failures and infertility, and encouraged the transmission of other sexually transmitted infections (Unemo, 2015). Figure 2 summarises the effects of antibiotic resistance.
The effects of ARB is not just limited to clinical effects alone – it could have a significant economic impact. Without effective antimicrobials, productivity levels would fall as workers take on extended periods of leave from work to recuperate (Smith, Keogh-Brown and Barnett, 2011). Caring for these patients places additional load on their community, families and the healthcare systems, in addition to the rippling societal and cultural impacts that arise from this. National outputs would reduce, more funds would need to be channeled towards healthcare. This is all but a tip of the iceberg describing an example of how antibiotic resistance can have a significant impact worldwide (Gandra, Barter and Laxminarayan, 2014).

Limitations of inventing new antibiotics to treat ARB
Inventing new antibiotics to treat ARB seems to be the easiest solution to tackle this problem. Unfortunately, this may not be a plausible solution.
The FDA approval of new antibacterial agents had decreased by 56% from 1998 to 2002, compared to 1983 to 1987 (Nature, 2008). Additionally, the mutation of bacteria is 300 mutations per population within 10 hours (Conly and Johnston, 2005). When considering our odds in the evolutionary race against bacteria, it is obvious that we are fighting a losing battle.
Generally, drug development itself is a challenging field. Ideally, a drug would only exert its effects on the desired cell after interfering with the microbe’s defense mechanism. However, in reality, there are many factors to be considered when designing a drug, particularly the pharmacokinetics and pharmacodynamics of the drug itself (Walker, 2004). Therefore, considering the mechanism of the drug alone would not guarantee its effectiveness. Moreover, drug development process is tedious. It requires the concerted efforts of personnel such as microbiologists and policymakers, years of clinical trials, before it is available for use.
Moreover, the antibiotics pipeline of new drugs has been drying up for years, as poor return on investment, short consumption periods to cure their target disease makes it unprofitable. Conversely, drugs which treat long term chronic illnesses are of greater interest to pharmaceuticals. An example would be the development of high profit biologics such as Rituxan and Humira, for the treatment of rheumatoid arthritis. This allows companies to generate more profit, making it a more lucrative market as compared to antibiotics.
In essence, the prospect of developing a new antibiotic drug is a potential solution to ARB. However, it is not a practical solution for the near future
Solutions in the post antibiotic era
Thankfully, all is not lost. There are numerous alternatives to antibiotics, such as bacteriophage therapy, though antibiotics has a greater efficacy (Allen, 2017). The advantage of these alternatives is that they only target the disease-causing bacterium. In contrast, antibiotics generally have collateral damage on the host’s beneficial commensal microbial communities. Further exploration and development of these alternatives in improving its reliability, deliverability and potency would allow them to be used as viable alternatives to antibiotics.

Moving forward, there are various steps that we can take to avert this antibiotics apocalypse. The first step is to improve sanitation and public health. Investing in better sanitation infrastructure, ensuring access to clean water supply, proper sewage and effluent treatment are paramount. This prevents the propagation, selection and spread of ARB and antibiotic resistant genes that are released into the environment and could be transmitted to humans (Jury et al, 2011).
Public education about antibiotic resistance, and how one can contribute to averting this crisis, are also important. As Dr Hsu Li Yang, associate professor and programme leader of the antimicrobial resistance (AMR) programme at the Saw Swee Hock School of Public Health in NUS, had sagaciously mentioned, “We are ‘addicted’ to antibiotics – it has become an integral part of human medicine and the livestock industry. The more antibiotics a person takes, the more pressure there is for the bacteria to evolve and become resistant.” (Khew, 2016). Although this problem of antibiotic resistance is acknowledged, there are still gaps in knowledge pertaining to antibiotic use and reasons for resistance (Darwish et al, 2014). Continuing and expanding public awareness about antibiotic resistance is therefore fundamental in promoting awareness and encouraging judicious use of antibiotics, to avoid subsequent emergence and spread of antibiotic resistance.
Presently, more antibiotics are used in agricultural production than on humans (WHO, 2012). ARB has been found in food, due to selective pressure from antibiotics usage during agricultural production, and cross-contamination during food processing (Verraes et al, 2013; Cabello, 2006). There is also a significant risk that ARB could enter the food chain, with food serving as vectors for the transmission to humans, particularly during the consumption of undercooked foods (Holmes et al, 2015). Thus, there should be strict regulations to restrict the use of antibiotics to therapeutic use only, imposed by organizations such as WHO, with sanctions in place to ensure compliance. .
There should also be greater engagement of other countries in global surveillance networks such as the Global Antimicrobial Resistance Surveillance System (GLASS) launched by WHO in 2015, as part of the Global Action Plan on AMR. GLASS allows for the sharing of data on AMR worldwide (WHO, 2017). This facilitates global, national, regional decision-making, and allows for a better global understanding of AMR, such as its economic implications. In addition, the information gathered about AMR can expedite research and development of alternatives to antibiotics.
Conclusion
In essence, exercising prudent and judicious use of antibiotics is key to tackling this crisis. Even if new antibiotics are discovered, the presence of ARB indicates that more expensive and/or toxic antibiotics are required. This translates to greater health care costs, morbidity and mortality. As Sun Tzu once said, “Know your enemy and know yourself and you can fight a hundred battles without disaster.” How can we prevent this apocalypse? We learn to strategically outsmart bacteria.
References
- Allen, H. K. 2017. Alternatives to antibiotics: Why and how. NAM Perspectives. Discussion Paper, National Academy of Medicine, Washington, DC. https://nam.edu/alternatives-to-antibiotics-why-and-how.
- Allen, H., Trachsel, J., Looft, T. and Casey, T. (2014). Finding alternatives to antibiotics. Annals of the New York Academy of Sciences, 1323(1), pp.91-100.
- Aminov, R. (2010). A Brief History of the Antibiotic Era: Lessons Learned and Challenges for the Future. Frontiers in Microbiology, 1(134).
- Bartlett, J., Gilbert, D. and Spellberg, B. (2013). Seven Ways to Preserve the Miracle of Antibiotics. Clinical Infectious Diseases, 56(10), pp.1445-1450.
- Cabello, F. C. (2006) Heavy use of prophylactic antibiotics in aquaculture: a growing problem for human and animal health and for the environment. Environmental Microbiology. 8(7), 1137–1144.
- Conly, J. and Johnston, B. (2005). Where are all the new antibiotics? The new antibiotic paradox. Canadian Journal of Infectious Diseases and Medical Microbiology, 16(3), pp.159-160.
- Darwish, D., Abdelmalek, S., Abu Dayyih, W. and Hamadi, S. (2014). Awareness of antibiotic use and antimicrobial resistance in the Iraqi community in Jordan. The Journal of Infection in Developing Countries, 8(05).
- Friedman, N., Temkin, E. and Carmeli, Y. (2016). The negative impact of antibiotic resistance. Clinical Microbiology and Infection, 22(5), pp.416-422.
- Gandra, S., Barter, D. and Laxminarayan, R. (2014). Economic burden of antibiotic resistance: how much do we really know?. Clinical Microbiology and Infection, 20(10), pp.973-980.
- Holmes, A. H., Moore, L. S., Sundsfjord, A., Steinbakk, M., Regmi, S., Karkey, A., Guerin, P. J. & Piddock, L. J. (2016) Understanding the mechanisms and drivers of antimicrobial resistance. The Lancet. 387(10014), pp.176–187.
- Jury, K., Khan, S., Vancov, T., Stuetz, R. and Ashbolt, N. (2011). Are Sewage Treatment Plants Promoting Antibiotic Resistance?. Critical Reviews in Environmental Science and Technology, 41(3), pp.243-270.
- Khew, C. (2017). Singapore to tackle bacterial resistance to antibiotics. [online] The Straits Times. Available at: http://www.straitstimes.com/singapore/health/spore-to-tackle-bacterial-resistance-to-antibiotics [Accessed 29 Aug. 2017].
- Nature. (2008). Mutation Rates and Antibiotic Resistance. [online] Available at: https://www.nature.com/scitable/topicpage/antibiotic-resistance-mutation-rates-and-mrsa-28360 [Accessed 29 Aug. 2017].
- OECD (2015) Antimicrobial Resistance in G7 countries and beyond: Economic Issues, Policies and Options for Action. Available from: http://www.oecd.org/els/health-systems/Antimicrobial-Resistance-in-G7-Countries-and-Beyond.pdf [Accessed 29 Aug. 2017].
- Smith, R., Keogh-Brown, M. and Barnett, T. (2011). Estimating the economic impact of pandemic influenza: An application of the computable general equilibrium model to the UK. Social Science & Medicine, 73(2), pp.235-244.
- The antibiotic alarm. (2013). Nature, 495(7440), pp.141.
- Unemo, M. (2015). Current and future antimicrobial treatment of gonorrhoea – the rapidly evolving Neisseria gonorrhoeae continues to challenge. BMC Infectious Diseases, 15(1).
- Verraes, C., van Boxstael, S., van Meervenne, E., van Coillie, E, Butaye, P., Catry, B., de Schaetzen, M. A., Van Hufferl, X., Imberechts, H., Dierick, K., Daube, G., Saegerman, C., de Block, J., Dewulf, J. & Herman, L. (2013) Antimicrobial resistance in the food chain: a review. International Journal of Environmental Research and Public Health. 10(7), pp.2643–2669.
- Walker, D. (2004). The use of pharmacokinetic and pharmacodynamic data in the assessment of drug safety in early drug development. British Journal of Clinical Pharmacology, 58(6), pp.601-608.
- WHO (2012). The evolving threat of antimicrobial resistance: options for action. Geneva, WHO. Available from: http://apps.who.int/iris/bitstream/10665/44812/1/9789241503181_eng.pdf [Accessed: 29 Aug. 2017].
- WHO. (2014). Antimicrobial resistance: global report on surveillance 2014. [online] Available at: http://apps.who.int/iris/bitstream/10665/112642/1/9789241564748_eng.pdf?ua=1 [Accessed 29 Aug. 2017].
- WHO. (2016) Antimicrobial resistance. Available from: http://www.who.int/mediacentre/factsheets/fs194/en/ [Accessed 29 Aug. 2017].
- World Health Organization. (2017). Global Antimicrobial Resistance Surveillance System (GLASS). [online] Available at: http://www.who.int/antimicrobial-resistance/global-action-plan/surveillance/glass/en/ [Accessed 30 Aug. 2017].