Hello Folks!
Earlier, we’ve seen how thermal pollution can drastically affect water quality and the marine creatures living in the affected water. We also see how climate change has increased the phenomenon of flash floods and hence, water pollution in an urban context.
Today, we will be looking at how climate change causes ocean acidification which is a growing concern that falls under the issue of ocean pollution.
As the word suggest, acidification means the average pH of our ocean is decreasing – becoming more acidic. How is that a concern and how serious is it?

Figure 1: Estimated change in seawater pH caused by human-created CO2 between the 1700s and the 1990s (Carbon, 2020)
In the span of the past 200 years since industrial revolution, our ocean’s pH has fallen on average by 0.1 unit as shown in Figure 1 . This may sound very little but… secondary school math will show you how drastic this numbers would represent.
To calculate pH:
pH= – log [H+], where [H+] = concentration of H+ ions.
Because of the logarithmic relationship, a fall of 0.1 pH unit translates to an approximate 30% increase in acidity of the water (PMEL CO2 – Carbon Dioxide Program, 2020)
How Does my Carbon Footprint Relates to this?
It all matters because this increased in acidity is due to the dissolution of excess CO2 into ocean waters and where does the CO2 come from? I guess we are know we are a culprit as part of this process.
How Does the Acidification Affects Marine Life?
To understand this, we have to understand some basic chemistry behind carbon dioxide reaction with water.
Firstly, carbon dioxide dissolves in water and reacts to form carbonic acid (fun fact: this is how you get coke/sparkling water).
Secondly, the carbonic acid can further react with the available carbonate ions in water to form a bicarbonate ion.
That is when the problem occurs. In a process called marine biogenic calcification, marine creatures that build shells and outer structures such as coral reefs, mollusca and sea urchines utilises carbonate ions to form calcium carbonate, which is the main constituent of these shells (Biogenic calcification – GEOMAR – Helmholtz-Zentrum für Ozeanforschung Kiel, 2020)
But by forming bicarbonate ions, what the carbonic acid does is to lower the availability of carbonate ions needed for the calcification process (Ocean acidification | National Oceanic and Atmospheric Administration, 2020).
That’s not the end of the story! If you look closely at the third equation, the formation of calcium carbonate from carbonate ions is reversible. In chemistry, the mechanism of a reversible reaction is governed by a principle called Le Chatelier’s principle (Clark, 2013). Sounds very complicated? Actually its not.To put in non-chemist perspective, when you are thirsty, what will you do? Drink water. When you feel cold, what will you do? Wear more layers of clothes.
Likewise, in a reaction system, if the system detects a fall in the amount of chemical substance in any parts of its system, the reaction will proceed in the direction to “correct” this loss by encouraging the increment of the substance.
Since the free carbonate ions in ocean are used up in the second equation by reacting with carbonic acid, in the third equation, the calcium carbonate will dissolve to increase the carbonate ions in the water so as to compensate this loss. In short, the shells of these marine creatures dissolve (see Figure 2) and we can expect a significant impact on the ecosystem, affecting up the food chain which can affect economic activities such as fisheries, aquaculture and tourism.
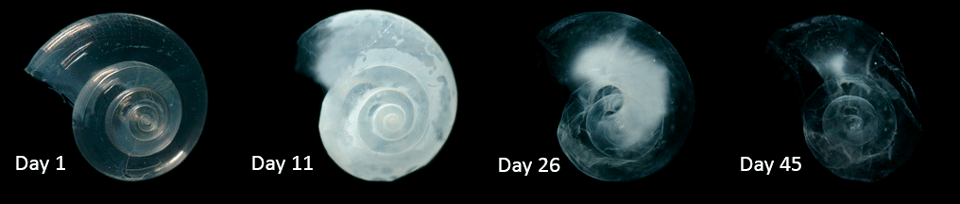
Figure 2: A mollusc shell dissolves under acidic conditions. The shell almost completely dissolves after 45 days when placed in seawater with pH and carbonate levels projected by models for the year 2100. (Liittschwager, 2011)
So, if you havent seen corals in your life, you really should see it one day before this ocean acidification gets worse because personally, I’ve seen some off the coast of Anambas Island (an Indonesian island in the middle of South China Sea) and they are really really beautiful! For the better of our future generation and these poor marine creatures, what we can really do is to reduce our carbon footprint and stop this trend of continuous carbon emission.
References:
1. Carbon, G., 2020. GLODAP: Global Ocean Data Analysis Project For Carbon | NCAR – Climate Data Guide. [online] Climatedataguide.ucar.edu. Available at: <https://climatedataguide.ucar.edu/climate-data/glodap-global-ocean-data-analysis-project-carbon> [Accessed 24 July 2020].
2. Pmel.noaa.gov. 2020. PMEL CO2 – Carbon Dioxide Program. [online] Available at: <https://www.pmel.noaa.gov/co2/story/A+primer+on+pH.> [Accessed 24 July 2020].
3. Geomar.de. 2020. Biogenic Calcification – GEOMAR – Helmholtz-Zentrum Für Ozeanforschung Kiel. [online] Available at: <https://www.geomar.de/en/research/fb2/fb2-bi/research-topics/biogenic-calcification/.> [Accessed 24 July 2020].
4. Noaa.gov. 2020. Ocean Acidification | National Oceanic And Atmospheric Administration. [online] Available at: <https://www.noaa.gov/education/resource-collections/ocean-coasts/ocean-acidification> [Accessed 24 July 2020].
5. Clark, J., 2013. Le Chatelier’s Principle. [online] Chemguide.co.uk. Available at: <https://www.chemguide.co.uk/physical/equilibria/lechatelier.html> [Accessed 24 July 2020].
6. Liittschwager, D., 2011. [online] Available at: <https://www.nationalgeographic.com/magazine/2011/04/ocean-acidification/> [Accessed 24 July 2020].

Leave a Reply