Contents
Fundamentals of Contact Electrification
Contact electrification is ubiquitous and has a wide range of applications or undesirable effects in our lives. Although the phenomenon has been observed since antiquity, the fundamental mechanism of contact electrification on insulating surfaces is still unclear. In practice, researchers and industries only rely on an empirical triboelectric series, which ranks the relative tendency of charging positively or negatively and is often unreliable, when considering the material effect from contact electrification. Hence, a fundamental understanding of contact electrification is desired when designing devices and selecting materials in various systems.
We are interested in deciphering the underlying principles governing contact electrification. In one of our recent publications, we proposed that the underlying principles of triboelectric series of polymers is based on the Lewis acidity/basicity of the repeat unit of the polymer. We successfully established a triboelectric series of 10 polymers and correlated its contact charging tendency to its Lewis basicity for every pair of materials. That implies ion transfer is the mechanism of contact electrification.1 In another study, we also found that there is a positive correlation of material transfer and charge transfer in contact electrification involving a soft material (e.g., polydimethylsiloxane, PDMS).2 Besides polymeric materials, we are also interested in contact electrification between inorganic insulating materials. Generally, it is expected that one surface acquires a positive charge, and the other surface acquires a negative charge after contact electrification. However, we found that some pairs of inorganic materials carry the same charge polarity (i.e., both charged positively or negatively) after contact electrification. It is due to the rapid interaction between the surface of the inorganic material and the atmosphere after contact. This anomalous charging behavior fundamentally changes our understanding of the design of devices related to contact electrification of inorganic materials.3
Fundamentals of Electrostatics
In addition to the phenomenon of the process of contact electrification, the properties of the static charge remaining (and also any electrostatic charge in/on a material by other charging methods) after the charging process is also of both fundamental and applicational importance. We are in particular interested in electrostatics in/on two types of materials – solid surfaces and liquids.
Charged surfaces have been used in applications such as electrophotography, electrostatic filtration/precipitation, electrostatic coating, energy generation (e.g., triboelectric nanogenerators), sensors, manipulation of multiphase fluids in microfluidic systems, electrical conductance modulation in heterogeneous nanostructured media, and actuators of nanomaterials. The ability to delicately control static charge on these surfaces is sought after in many of these applications. It was commonly believed that surface static charge is a property of the material, and a change of charge requires discharging and/or recharging the material, which is often complicated and undesirable. We are the first to report that the surface charge of a material is fundamentally a variable which depends on the shape of the material. The change in charge could be due to a dynamic exchange of charge from the material to the surrounding atmosphere as the shape changes via the reversible ionization and deposition of air molecules. A force sensor with a wide force detection range was built based on this phenomenon.4 The static charge of an insulating surface can also change reversibly by controlling the distance to another charged insulated surface.5 The fundamental relationship between geometry, environment and electrostatics via chemistry is important for the broad range of applications related to the charge of flexible materials.
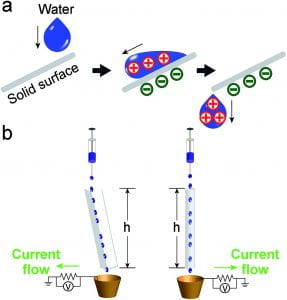
Our group also established methods of electrostatically charging liquids. Charged liquids have a handful of properties that make applications such as electrostatic coating, dispersion of chemicals, mass spectrometry, fabrication of micro- and nanoscale materials (e.g., polymer scaffolds, crystals, and metallic particles), and control of chemical reactions possible. We successfully developed a simple and general strategy to flexibly charge various types of liquids. By simply flowing the liquid on a previously contact-charged solid surface, the charge is transferred from the solid surface to the bulk of liquid as molecular ions, and hence the liquid is charged. This strategy successfully charges water, and more importantly, insulating non-polar organic liquids, which are difficult to charge even by applying high electric potential. We also fabricated permanently charged electric-field and magnetic-field responsive particles for the first time by polymerizing the mixture of liquid monomers charged with this method and magnetic nanoparticles.6, 7
Materials Considerations
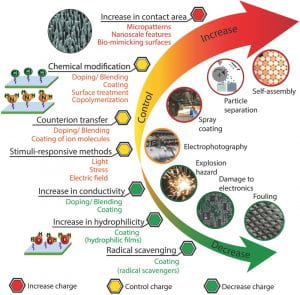
Besides the fundamental mechanisms of contact electrification and electrostatics, material based strategies are also vital when trying to increase, control or decrease surface charge generated by contact electrification. These strategies include chemical modification of material surfaces, chemical modification of bulk materials, modification of surface morphology, and stimuli-responsive methods. They are summarized in our previous review article on Advanced Materials.8
Application 1: Fabricating Non-charging Surfaces
One important application from the knowledge of electrostatics and charge is fabricating non-charging surfaces. Surface charge causes slight annoyances in many everyday activities, such as the sticking of clothes onto each other while drying and the experience of a shock when one touches a doorknob in dry weather. Electrostatic adhesion of dust particles on surfaces leads to problems in both daily life (e.g., dust sticking on screens of computers and mobile phones) and industries (e.g., fouling and preventing homogenous mixing). Electrostatic discharge (ESD) can occur if excessive amount of charge accumulates and poses a severe explosion hazard.
To eliminate the problems caused by surface charge, we developed several methods to fabricate non-charging insulating surfaces. One method is to coat radical-scavenging chemicals on the surface of materials. By removing the radicals co-generated with the charges during contact electrification, the charge of the surfaces was greatly reduced.9 Another general materials strategy to fabricate non-charging insulating surface is to mix two different types of materials with opposite contact charging tendency based on our understandings of contact electrification and triboelectric series. By either copolymerizing two polymers,10 or physically mixing particles of one type of material into the bulk pre-polymer liquid of the other type,11 a zero-charging point can be determined under different environmental and operational conditions. A truly non-charging surface is thus obtained. This technology is patented, and more details can be found in Application: Non-charging Technology.
Application 2: Energy Harvesting
Clean and renewable energy is one of the most needed resources in the current world. One proven form of renewable energy is the gravitational energy of water. Utilizing both the advantages of contact electrification (directly converts to electrical energy) and water (liquid state, high ion mobility, readily charged against other solids), our group developed a novel strategy to collect electricity from falling water droplets via contact with solid materials with up to 4% energy conversion efficiency.12 This method is clean, renewable, technically simple and inexpensive to produce, and potentially scalable. Find out more information in Application: Energy Harvesting.
- Zhang, X.; Chen, L.; Jiang, Y.; Lim, W.; Soh, S.* Rationalizing the Triboelectric Series of Polymers. Chemistry of Materials 2019, 31, 1473-1478.
- Pandey, R. K.; Kakehashi, H.; Nakanishi, H.*; Soh, S.* Correlating Material Transfer and Charge Transfer in Contact Electrification. The Journal of Physical Chemistry C 2018, 122, 16154-16160.
- Fang, Y.; Chen, L.; Sun, Y.; Yong, W. P.; Soh, S.* Anomalous Charging Behavior of Inorganic Materials. The Journal of Physical Chemistry C 2018, 122, 11414-11421.
- Pandey, R. K.; Ao, C. K.; Lim, W.; Sun, Y.; Di, X.; Nakanishi, H.*; Soh, S.* The Relationship between Static Charge and Shape. ACS Central Science 2020, 6, 704-714.
- Pandey, R. K.; Sun, Y.; Nakanishi, H.*; Soh, S.* Reversible and Continuously Tunable Control of Charge of Close Surfaces. The Journal of the Physical Chemistry Letters 2017, 8, 6142-6147.
- Lim, K. H.; Sun, Y.; Lim, W. C.; Soh, S.* Charging Organic Liquids by Static Charge. Journal of the American Chemical Society 2020, published online.
- Sun, Y.; Huang, X.; Soh, S.* Solid-to-Liquid Charge Transfer for Generating Droplets with Tunable Charge. Angewandte Chemie-International Edition 2016, 55, 9956-9960. (Hot Paper)
- Chen, L.; Shi, Q.; Sun, Y.; Nguyen, T.; Lee, C.*; Soh, S.* Controlling Surface Charge Generated by Contact Electrification: Strategies and Applications. Advanced Materials 2018, 30, 1802405.
- Fang, Y.; Gonuguntla, S.; Soh, S.* Universal Nature-Inspired Coatings for Preparing Noncharging Surfaces. ACS Applied Materials & Interfaces 2017, 9, 32220-32226.
- Zhang, X.; Huang, X.; Kwok, S. W.; Soh, S.* Designing Non-charging Surfaces from Non-conductive Polymers. Advanced Materials 2016, 28, 3024-3029.
- Zhang, X.; Ao, C. K.; Soh, S.* Nonconductive Noncharging Composites: Tunable and Stretchable Materials for Adaptive Prevention of Charging by Contact Electrification. ACS Applied Materials & Interfaces 2020, 12, 5274-5285. (Invited Article for the Young Investigator Forum)
- Sun, Y.; Huang, X.; Soh, S.* Using the Gravitational Energy of Water to Generate Power by Separation of Charge at Interfaces. Chemical Science 2015, 6, 3347-3353.